
PharmaShots Weekly Snapshots (March 17, 2025 – March 21, 2025)
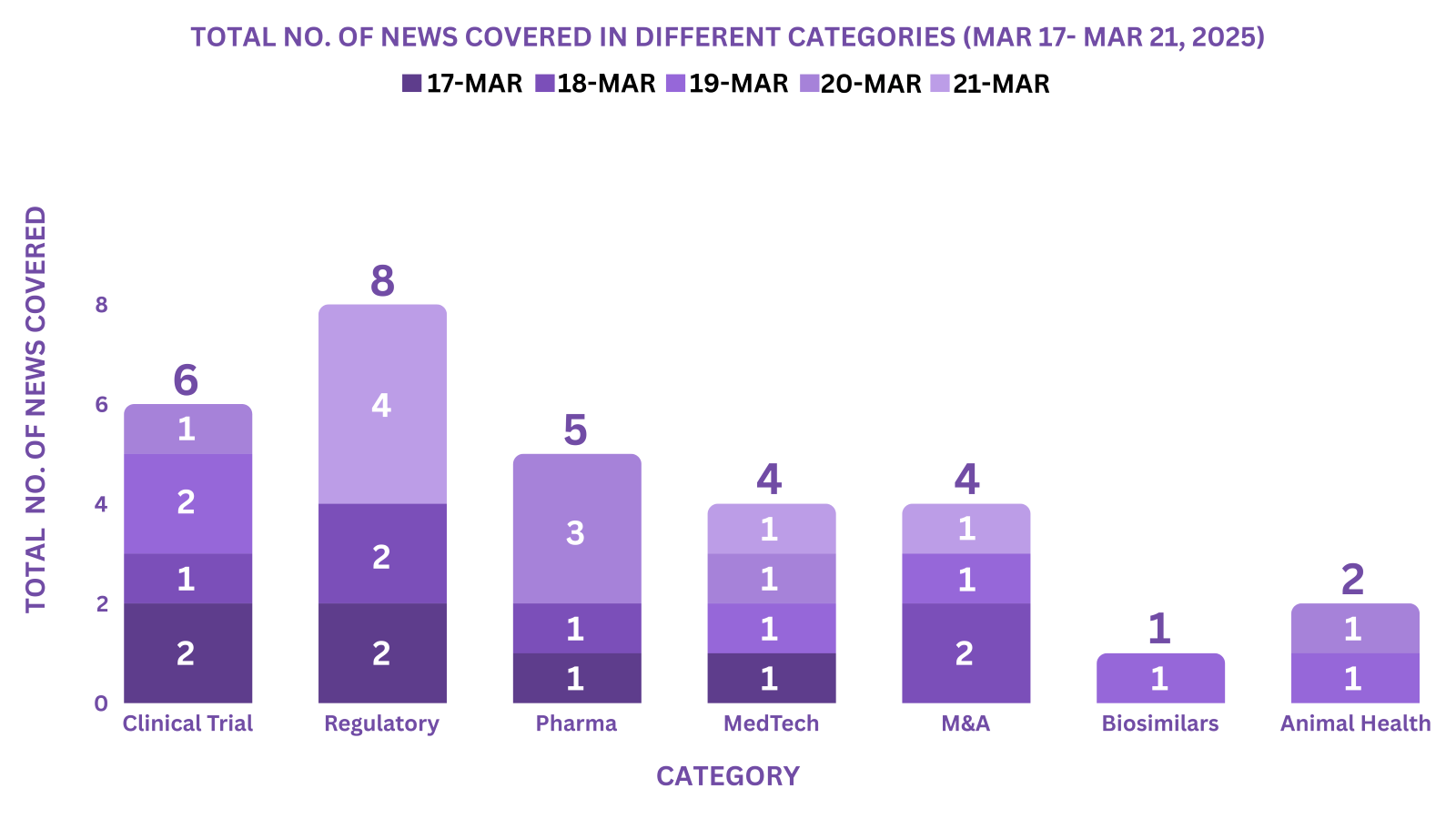
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Biosimilars & Animal Health. Check out our full report below:

AbbVie Reports P-III (MIRASOL) Trial Data of Elahere for FRα-Positive Platinum-Resistant Ovarian Cancer (PROC)
Read More: AbbVie
Incyte Reports Topline Data from P-III (STOP-HS1 & STOP-HS2) Trials of Povorcitinib (INCB054707) for Hidradenitis Suppurativa (HS)
Read More: Incyte
Amgen Reveals P-III (MINT) Study Data of Uplizna (Inebilizumab-cdon) to Treat Generalized Myasthenia Gravis (gMG)
Read More: Amgen
AstraZeneca Reports P-III (CALYPSO) Trial Data of Eneboparatide for Chronic Hypoparathyroidism (HypoPT)
Read More: AstraZeneca
Innovent and HUTCHMED Report P-II/III (FRUSICA-2) Trial Data of Sintilimab + Fruquintinib for Metastatic Renal Cell Carcinoma (RCC)
Read More: Innovent and HUTCHMED
Novartis Reports P-III (STEER & STRENGTH) trials data of OAV101 IT for Spinal Muscular Atrophy
Read More: Novartis

BMS Reports the EC’s Approval of Breyanzi to Treat R/R Follicular Lymphoma
Read More: BMS
AstraZeneca Reports the EC’s Approval of Imfinzi to Treat Limited-Stage Small Cell Lung Cancer (LS-SCLC)
Read More: AstraZeneca
Bayer Reports the US FDA’s sNDA Acceptance & Priority Review of Finerenone for Heart Failure (HF)
Read More: Bayer
Innovent Reports the NMPA Approval of Sycume for Thyroid Eye Disease
Read More: Innovent
Alnylam Pharmaceuticals Reports the US FDA’s Approval of Amvuttra (Vutrisiran) to Treat ATTR Amyloidosis with Cardiomyopathy (ATTR-CM)
Read More: Alnylam Pharmaceuticals
Novartis Reports the US FDA’s Approval of Fabhalta (Iptacopan) to Treat C3 glomerulopathy
Read More: Novartis
Johnson & Johnson Reports the US FDA’s Approval of Subcutaneous & Intravenous Tremfya for Active Crohn’s Disease (CD)
Read More: J&J
HUTCHMED Receives NMPA’s Conditional Approval for Tazverik to Treat R/R Follicular Lymphoma
Read More: HUTCHMED

AstraZeneca and Alteogen Enter an Exclusive License Deal for ALT-B4 to Develop & Market Subcutaneous Formulations of Multiple Oncology Drugs
Read More: AstraZeneca and Alteogen
NANOBIOTIX and Janssen Amend Partnership to Develop and Commercialize JNJ-1900 (NBTXR3)
Read More: NANOBIOTIX and Janssen
Oxford BioTherapeutics and Roche Collaborate to Discover Next-Gen Biologics for Cancer Treatment
Read More: Oxford BioTherapeutics and Roche jnj
Servier Collaborates with Black Diamond Therapeutics to Advance BDTX-4933 for Solid Tumors
Read More: Servier and Black Diamond Therapeutics
Sanofi to Acquire Dren Bio’s Bispecific Antibody DR-0201 for ~$1.9B
Read More: Sanofi and Dren Bio

Perfuze Secures the US FDA’s 510(k) Clearance for its Zipline Access Catheters
Read More: Perfuze
bioMérieux Reports the US FDA’s 510(k) Clearance of VITEK COMPACT PRO for Pathogen Detection & Antibiotic Susceptibility Testing
Read More: bioMérieux
Orthofix Medical Receives US FDA’s 510(k) clearance & European CE Mark for its TrueLok Elevate Transverse Bone Transport (TBT) System
Read More: Orthofix Medical
Moon Surgical Reports US FDA’s 510(k) Clearance of ScoPilot to Improve Intraoperative Capability
Read More: Moon Surgical

AstraZeneca to acquire EsoBiotec for ~$1B
Read More: AstraZeneca and EsoBiotec
Endo & Mallinckrodt Pharmaceuticals to Form a Combine Entity with $6.7B Cash-and-Stock
Read More: Endo & Mallinckrodt Pharmaceuticals
Taiho Pharmaceutical to Acquire Araris Biotech for ~$1.1B
Read More: Taiho Pharmaceutical and Araris Biotech
Paratek Pharmaceuticals to Acquire Optinose for ~$330M, Expanding its Commercial Portfolio
Read More: Paratek Pharmaceuticals and Optinose

Elanco and WEDterinary Collaborate to Develop Novel Therapies for Pets
Read More: Elanco and WEDterinary
ELIAS Animal Health Reports USDA’s Full Approval of ELIAS Cancer Immunotherapy (ECI) for Canine Osteosarcoma
Read More: ELIAS Animal Health

Alvotech and Dr. Reddy’s Report the US FDA’s BLA Acceptance for AVT03 (Biosimilar, Prolia and Xgeva)
Read More: Alvotech and Dr. Reddy’s
Related Post: PharmaShots Weekly Snapshots (March 10, 2025 – March 13, 2025)
Tags

Ridhi is an avid secondary researcher who follows trends in the biopharmaceutical and healthcare sectors to curate engaging content for the global audience. She works as a news editor at PharmaShots and loves to read books and explore new destinations.














